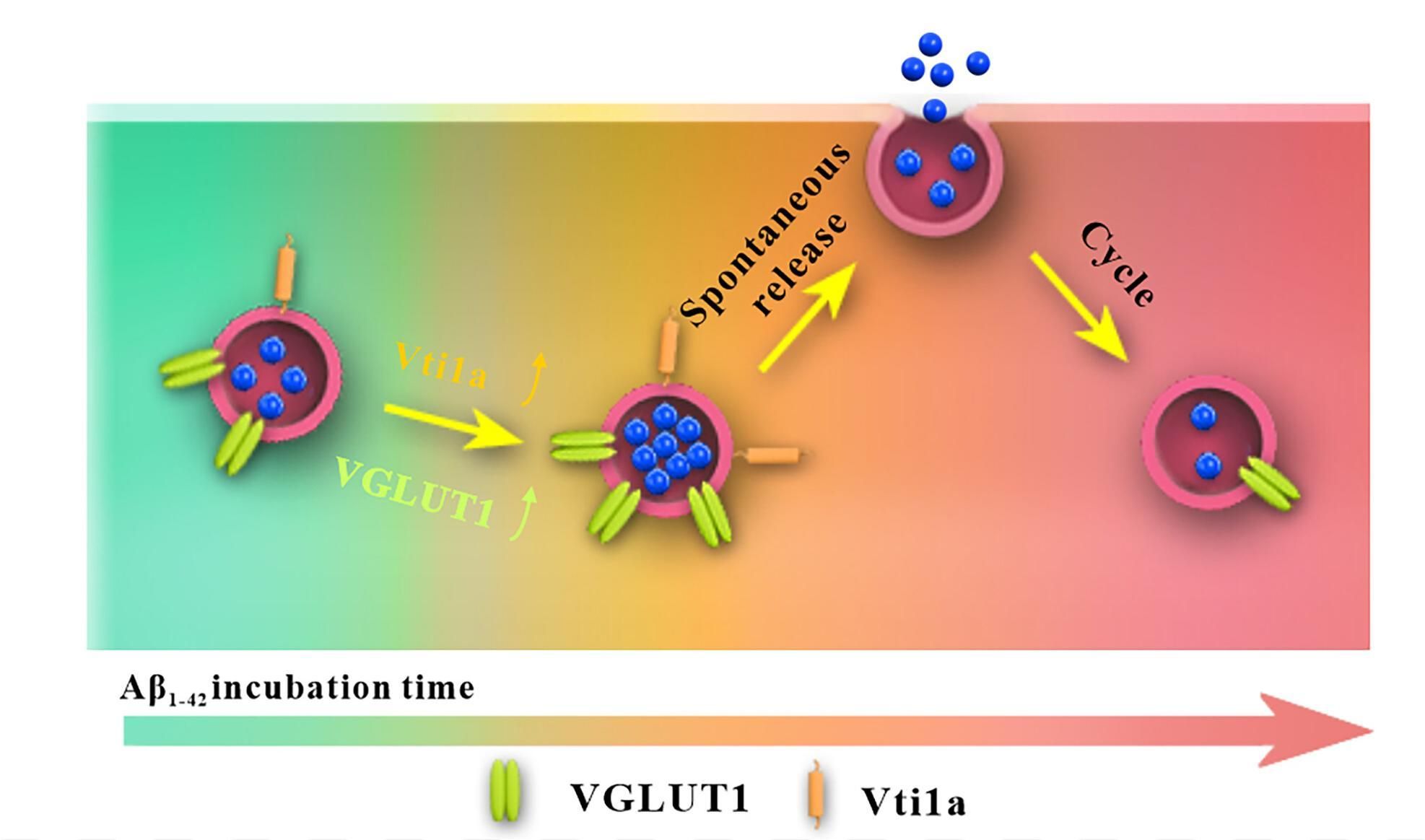
Abstract: Glutamate (Glu) is the major excitatory transmitter in the nervous system. Impairment of its vesicular release by β-amyloid (Aβ) oligomers is thought to participate in pathological processes leading to Alzheimer’s disease. However, it remains unclear whether soluble Aβ42 oligomers affect intravesicular amounts of Glu or their release in the brain, or both. Measurements made in this work on single Glu varicosities with an amperometric nanowire Glu biosensor revealed that soluble Aβ42 oligomers first caused a dramatic increase in vesicular Glu storage and stimulation-induced release, accompanied by a high level of parallel spontaneous exocytosis, ultimately resulting in the depletion of intravesicular Glu content and greatly reduced release. Molecular biology tools and mouse models of Aβ amyloidosis have further established that the transient hyperexcitation observed during the primary pathological stage is mediated by an altered behavior of VGLUT1 responsible for transporting Glu into synaptic vesicles. Thereafter, an overexpression of Vps10p-tail-interactor-1a, a protein that maintains spontaneous release of neurotransmitters by selective interaction with t-SNAREs, resulted in a depletion of intravesicular Glu content, triggering advanced-stage neuronal malfunction. These findings are expected to open perspectives for remediating Aβ42-induced neuronal hyperactivity and neuronal degeneration.
DOI: 10.1073/pnas.2219994120