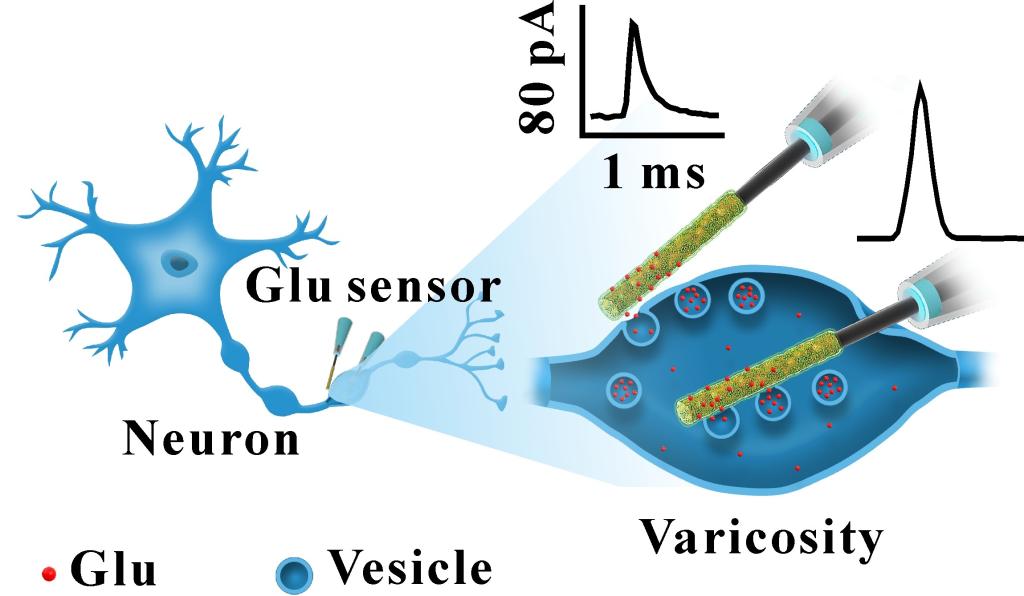
ABSTRACT:Glutamate (Glu) is a crucial fundamental excitatory neurotransmitter released through vesicular exocytosis in the central nervous system. Hence, quantitative measurements of intravesicular Glu and of transient exocytotic release contents directly from individual living neurons are highly desired for understanding the mechanisms (full or sub-quantal release?) of synaptic transmission and plasticity. However, this could not be achieved so far due to the lack of adequate experimental strategies relying on selective and sensitive Glu nanosensors. Herein, we introduce a novel electrochemical Glu nanobiosensor based on a single SiC nanowire prone to selectively measure in real-time Glu fluxes released via exocytosis by large Glu vesicles (ca. 125 nm diameter) present in single hippocampal axonal varicosities as well as their intravesicular content before exocytosis. Combination of these two series of measurements revealed a sub-quantal release mode in living hippocampal neurons, viz., only ca. one third to one half of intravesicular Glu molecules are released by individual vesicles during exocytotic events. Importantly, this fraction remained practically the same when hippocampal neurons were pretreated with L-Glu-precursor L-glutamine, while it significantly increased after zinc treatment, although in both cases the intravesicular contents were drastically affected. By affording quantitative measurements of intravesicular Glu inside living neurons and of its sub-quantal release fractions this work is opening new possibilities for investigating in situ and in time the regulatory mechanisms of glutamatergic neurotransmission and plasticity.
DOI:10.1002/anie.202100882